A Breakthrough Stem Cell Therapy Opens New Paths for Autoimmune Disease Patients
The world has seen a four-fold rise – in just 30 years — in the number of Type 2 Diabetes cases (880 million according to the WHO 2024 report1) so new drugs (Ozempic, Wegovy) have emerged as novel long-term treatments.
DATA SNAPSHOT-USA: According to the CDC, about 34 million Americans have Type 2 Diabetes, while about 1.6 million are estimated to have Type 1 Diabetes.
Meanwhile, a quieter, more important breakthrough has also emerged for treating Type 1 Diabetes and other autoimmune conditions (with 50 million US cases). That groundbreaking work comes from Throne Biotechnologies of Paramus, NJ (US).
Throne’s innovation: Use cord blood stem cells to probe new ways to significantly treat Type 1 Diabetes plus a growing list of autoimmune conditions — from Long COVID to Crohn’s.
A Game-Changing Stem Cell Treatment for Autoimmune Conditions?
Autoimmune diseases occur when the body’s immune system mistakenly attacks healthy cells, creating chronic inflammation and long-term damage. Conventional treatment simply targets symptoms, using steroids to suppress immune activity.
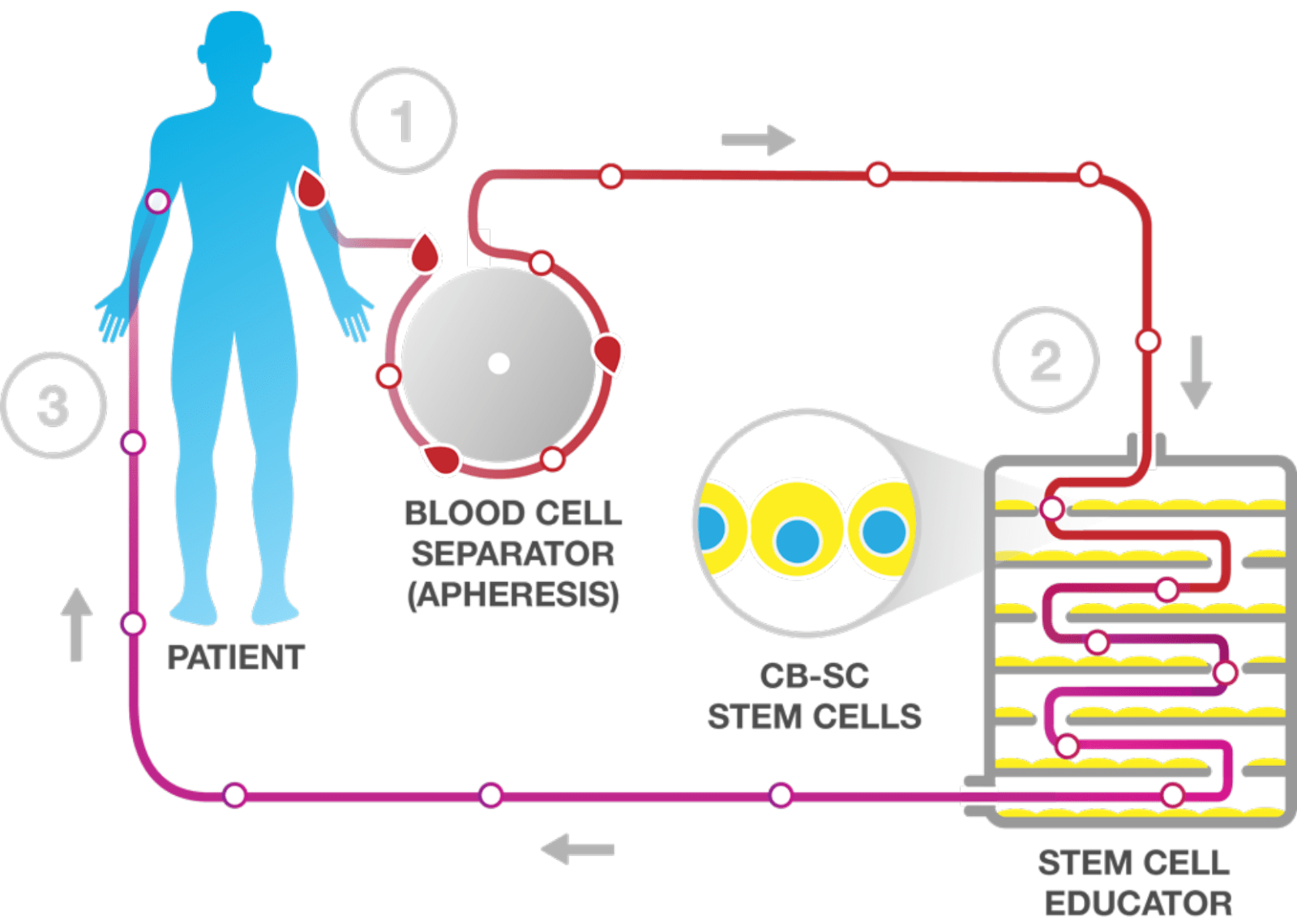
Throne’s approach, however, uses its Stem Cell Educator (SCE) Therapy (image below). This deploys human Cord Blood Stem Cells (CB-SCs) to recruit the patient’s own immune system, repairing and resetting that immune system and recreating its balance. As a clinical stage company, Throne Biotechnologies is also an FDA-designated Regenerative Medicine innovator, a rare and key recognition for its continuing success in treating autoimmune disorders.
Developed by Dr. Yong Zhao, MD, Ph D and Founder and CEO of Throne, SCE Therapy uses human cord blood stem cells (CB-SC) to modulate the vital immune function. By design, the process helps CB-SCs reprogram defective immune cells, targeting the root cause of autoimmune and chronic inflammation-associated disorders.
“Our CB-SC can target different compartments of the immune system, educating and correcting their dysfunctions at a fundamental level,” says Dr. Zhao. “This therapy has the potential to transform autoimmune treatments, and our 20-plus years of clinical research has demonstrated this.”
The Stem Cell Educator Process
Outcomes from Throne’s recent clinical trials show that SCE Therapy successfully creates unexpected new paths to relief and healing for long-suffering patients:
- Type 1 Diabetes – Cord blood stem cells stop the autoimmunity and regenerate insulin–producing islet β cells, thereby slowing (or stopping) disease progression.
- Long COVID – Many Long COVID patients often experience lingering immune dysfunction and sleeplessness. CB-SC help reduce inflammation, repair damaged tissues, and restore immune function — giving patients long-lasting symptom relief.
- Alopecia Areata – The Stem Cell Educator regrow hairs, often all over the body.
- Crohn’s Disease – Stem cells help heal the gut lining, reduce inflammation and improve digestive health (leaving behind lifelong drugs and invasive treatments).
With 20 years of progressive trials delivering promising outcomes, Dr. Zhao’s SCE therapy is proven to create healing for many autoimmune patients. Early and recent trials show that this therapy successfully modulates and resets patients’ immune systems, much like a blood transfusion refreshes a body in distress. The therapy uses apheresis, familiar to us from seeing that process working in kidney dialysis.
Put simply, Throne’s treatment goal, as Dr. Boris Veysman, Throne’s Medical Director says, is to modulate a body’s immune system so that the body “forgets” its autoimmunity. Patients treated with SCE Therapy report a “night and day” difference after 2 months. (See more patient comments at the Reuters film link below.)
Glancing Ahead
CEO Dr. Zhao has high confidence in this therapy’s record of efficacy as it moves through clinical trials. In 2024, the company also received the FDA’s Regenerative Medicine Advanced Therapy (RMAT) designation, a distinction granted only to a select number of pioneering therapies. This underscores the FDA’s recognition of SCE’s potential to address the unmet medical needs in regenerative medicine. The RMAT status has one more benefit: It expedites development and regulatory support, accelerating the time when SCE therapy is available to patients in Europe and beyond.
Given its clinical outcomes, Throne sees growing potential for SCE Therapy becoming the new standard of care for immune-mediated conditions.
To watch the Reuters Global Health Series film featuring Throne Biotechnologies work – and the responses of patients – please go here.
1 Note: Read here the World Health Organization’s (WHO) 2024 report on global diabetes.
Find out more about Global Health campaign