MEET EDISON: Non-Invasive Tumor Destruction
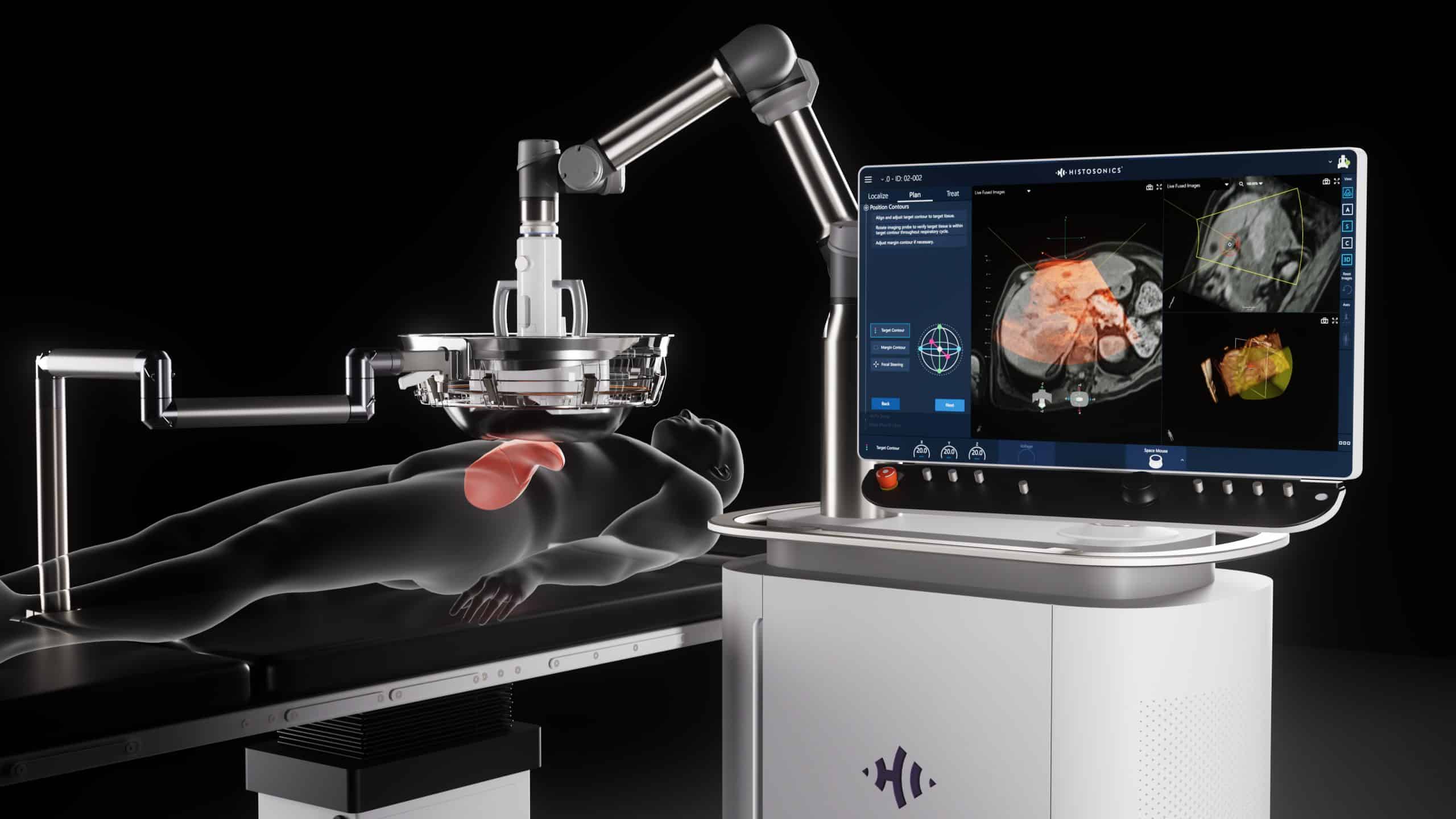
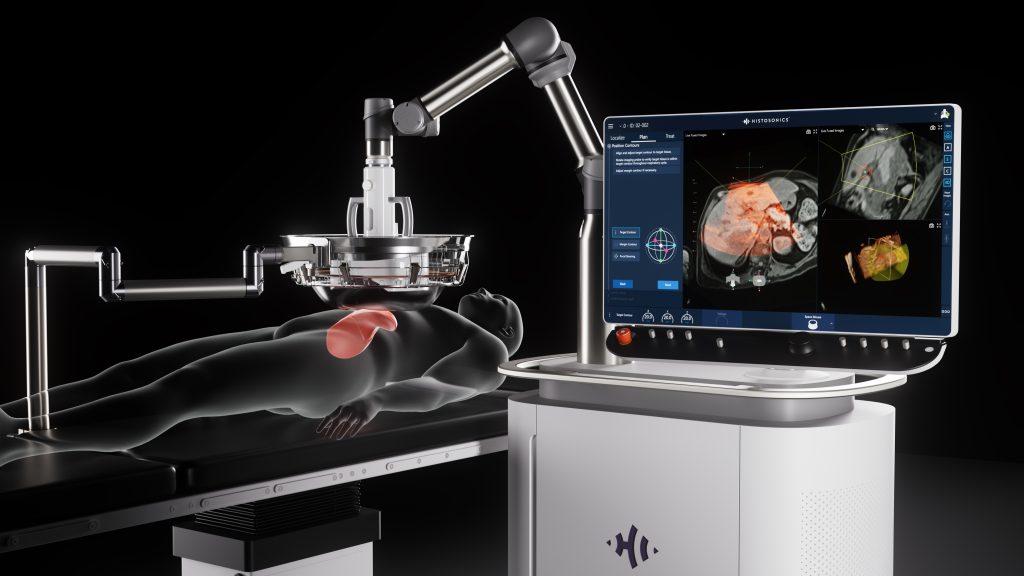
HistoSonics developed the Edison System as a new category of care, delivering non-invasive histotripsy treatment to destroy tumors.
Mission
By 2050, the CDC expects the total number of new patients diagnosed with liver tumors in the U.S. to increase by almost 50%.1 Current treatments for patients with liver tumors are often invasive, can damage healthy tissue, carry associated risks such as toxicity and often have an unfavorable quality of life impact for patients. HistoSonics is a new platform in human health, focused on improving the lives of patients with significant diseases, starting in cancer.
Technology
HistoSonics developed the Edison System as a new category of care, overcoming the limitations of existing treatment modalities. The Edison System delivers novel histotripsy treatment with a collective set of patient benefits never seen before in cancer care:
- Completely non-invasive
- Single therapy session with the ability to treat multiple tumors
- No radiation-related toxicities
- Non-ionizing and non-thermal mechanism of action
- Preserve critical anatomy like larger blood vessels and bile ducts
- Outpatient procedure enabling most patients to return home same day
- Treatment sites tend to resorb and recover quickly
The Science of Histotripsy
Histotripsy is a form of focused ultrasound that uses high amplitude, very short pulses designed to mechanically liquefy tumors within the physician-defined Planned Treatment Volume. As ultrasound waves converge on the targeted tissue, the rapid expansion and collapse of naturally occurring gas micro-bubbles forms a bubble cloud which induces instantaneous cell death and leaves behind an acellular lysate, tending to preserve antigenic proteins.2
Patient Impact
“No pain, no intrusion into my body, no nothing. I expected something more than being able to go home the day after and get fish and chips,” states Sheila after her histotripsy procedure. Being completely non-invasive with very minimal side effects has been a game-changer for patients around the world. Histotripsy continues to deliver a newfound hope to many patients who are looking for more innovative treatment options that have less side effects, who do not qualify for other treatments or who are too sick to receive more invasive or toxic treatments.
Ongoing Trials and Research
Founded in 2009, HistoSonics received Food and Drug Administration clearance in October 2023 for treating liver tumors with histotripsy. Doctors are now using it on tumors that originate in the liver or have spread to the organ.
In a recent study published in Radiology, histotripsy was evaluated and found to be both safe and effective by successfully meeting both study goal endpoints.
In order to expand the Edison System platform to reach more patients seeking non-invasive treatment options, HistoSonics is currently enrolling in a #HOPE4KIDNEY Pivotal Trial. #HOPE4KIDNEY is designed to evaluate the safety and technical success of the Edison System in targeting and destroying targeted primary renal tumors,
HistoSonics will also be initiating a novel Master Study design by the end of the year. The study and post market clinical program, called BOOMBOX, aims to collect data across all clinical use cases, and liver tumor pathologies, observing the use of histotripsy across all stages of liver disease.
REFERENCE:
- Weir HK, Thompson TD, Stewart SL, White MC. Cancer Incidence Projections in the United States Between 2015 and 2050. Prev Chronic Dis 2021;18:210006. DOI: http://dx.doi.org/10.5888/pcd18.21000
- Weir HK, Thompson TD, Stewart SL, White MC. Cancer Incidence Projections in the United States Between 2015 and 2050. Prev Chronic Dis 2021;18:210006. DOI: http://dx.doi.org/10.5888/pcd18.21000
Find out more about Global Health campaign